Pipe reactors in Ammonium-Nitrate (AN) plants suffer from short lifetimes due to serious corrosion and erosion issues. NobelClad’s DetaPipe™ coupled with its highly used and recommended DetaClad™ solution could provide a unique opportunity to address the problem faced by licensors and end-users in the pipe-reactors of ammonium-nitrate plants. The NobelClad solution provides higher safety and reliability standards and less downtime and maintenance leading to an attractive pay-back time. NobelClad’s DetaPipe™ has several possibilities for usage in mining, fertilizer, and other chemical applications.
Presently, NobelClad is encouraging end-users and licensors to contact us to jointly test sample spools in your critical applications.
Introduction Ammonium-Nitrate Process
Often seen in the production of inorganic fertilizers, a pipe reactor is an acid-base reaction vessel that uses reaction heat as the primary method of drying. This process reduces the burden on the dryer and can significantly decrease plant energy costs.
The pipe reactor accepts phosphoric, sulfuric or nitric acid in one side of the pipe, and gaseous or liquid ammonia is sparged into the reaction chamber. This results in either ammoniated phosphate, sulfate or nitrate, a hot “melt” of superheated product [1].
The contained heat of the reaction contributes to the heat requirement for moisture removal of the granulated material. This reduces the dryer fuel requirements for the plant.
Pipe reactors are popular equipment items in several ammonium nitrate process technologies. Ammonia gas reacts with heated nitric acid releasing heat which evaporates a significant part of the water fraction leading to a concentrated ammonium-nitrate (AN) solution. Fig. 1 shows the flow scheme of the Grande Paroisse (now Casale) AN process.
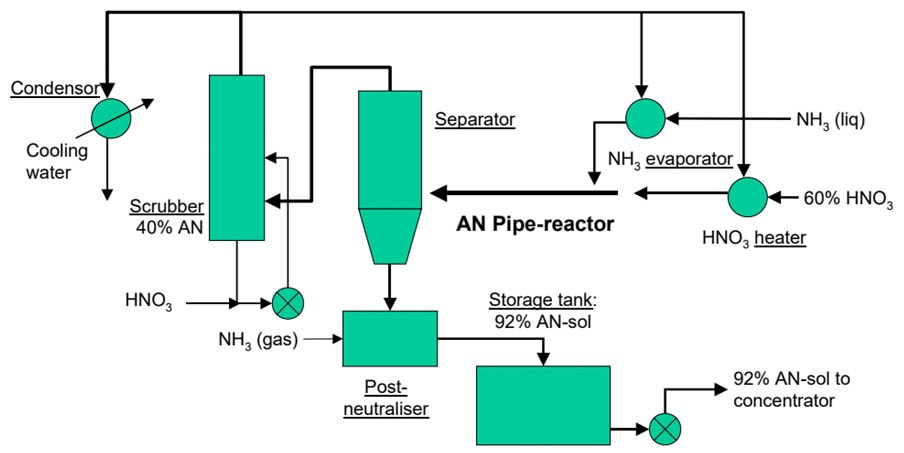
Figure 1: Grande Paroisse Ammonium-Nitrate process scheme
The ammonia flow rate is measured in the liquid phase and then the ammonia is vaporized and superheated up to 90°C. Heat is provided by the condensation from process steam generated by the neutralization reaction. Hot gaseous ammonia is fed into the pipe reactor.
The nitric acid flow rate is measured and controlled through ratio control related to the ammonia flow. Nitric acid is heated in a heat exchanger fed with process steam to increase the ammonium nitrate concentration.
The instantaneous reaction starts as soon as nitric acid and ammonia are mixed and goes on all along the pipe reactor. Pressure decreases from the reactor head (4~7 abs. bar) to the separator tank ( ~1 abs. bar). At the pipe reactor outlet, the Ammonium-Nitrate-Solution (ANS) and process steam are split in a separator.
The ANS flows by gravity from the separator tank into the buffer tank where a small flow of gaseous ammonia is fed to automatically adjust the pH of the solution.

Figure 2: Grande Paroisse Pipe Reactor at DSM Geleen (now OCI)
Pipe Reactor Issues and Its Causes
A main advantage of a pipe reactor is its operating flexibility, it can be operated in a wide capacity range. For example, the above-referred pipe reactor was designed for a capacity of 1650 te/d and has been operating within a range of 700 and 2000 te/d. This range is achieved through one single pipe reactor (DN 200 mm – length 9 m).
However, in the stainless steel 304L pipe reactor, corrosion at a rate of approx. 12 mm/year takes place in a zone of some 1 meter in length, close to the nitric acid inlet. Besides possible erosion phenomena, the following corrosion mechanism is likely to happen in this turbulent environment.
The already heated nitric acid plus the exothermic heat developed from the reaction between ammonia and nitric acid creates alternating wet-dry zones (condensation and re-evaporation).
The anodic reaction is: Me ↔ Men+ + ne–
While the cathodic reaction is: NO3- + 4H+ + 3e– ↔ NO + 2H2O (Eo = + 940 mV)
In these alternating wet-dry zones, dichromic acid will be formed and the above cathodic nitrate reduction reaction will be overruled by: Cr2O72- + 14H+ + 6e– ↔ 2Cr3+ + 7H2O (Eo = + 1330 mV)
The higher equilibrium potential of this redox reaction will shift the corrosion potential further in the trans-passive area of the polarization curve leading to high corrosion rates of stainless steels.
With the target of better corrosion resistance, titanium has been used for these pipe reactors. A passive layer of titanium oxide provides corrosion resistance. However, if this protective layer is broken, the exposed metal surface will oxidize, and the resulting combustion process can
be highly exothermic. Therefore, care is needed when considering titanium for this application. To establish the safety of the use of titanium for nitric acid spargers, appropriate testing or assessment has been carried out by several companies. The details of such work do not seem to be available in the open literature for nitric acid reactions, special care is advisable when urea off gases are used as a source of ammonia. The presence of contamination with incompatible substances such as iron should be avoided [2]. Besides the above-mentioned safety risks, titanium pipe reactors also show a limited lifetime of six months maximum as the corrosion resistance of titanium in nitric acid concentrations is limited to 40-60% [3].
Solutions
Grande Paroisse has operating experience for more than five years in two of its own plants (now owned by Agrofert, earlier Borealis). There, the corrosion zone of the pipe reactor is also made of SS304L, but fitted with an internal lining. Those pipe reactors can operate for more than two years without any corrosion issues.
In the DSM-Agro (now OCI) plant, it is preferred to maintain the equipment as simply as possible, and every six months the pipe reactor is changed and repaired.
NobelClad, a DMC Global Company, headquartered in Colorado was approached by some of the end-users.
NobelClad is an industry leader in explosion welding (EXW), which is a solid-state welding technology for the manufacture of large clad metal plates, which are sold under its trademark DetaClad™. The technology was discovered around 1960. Explosion clad products are used extensively, worldwide in the manufacture of corrosion-resistant process equipment as well as other bi-metallic applications. The cladding metal alloy can be selected for optimum corrosion performance. The base metal alloy can be specified to optimize strength, fabricability, and cost. The high strength, durable explosion weld allows for the construction of chemical process industry equipment exhibiting the beneficial features of both [4].
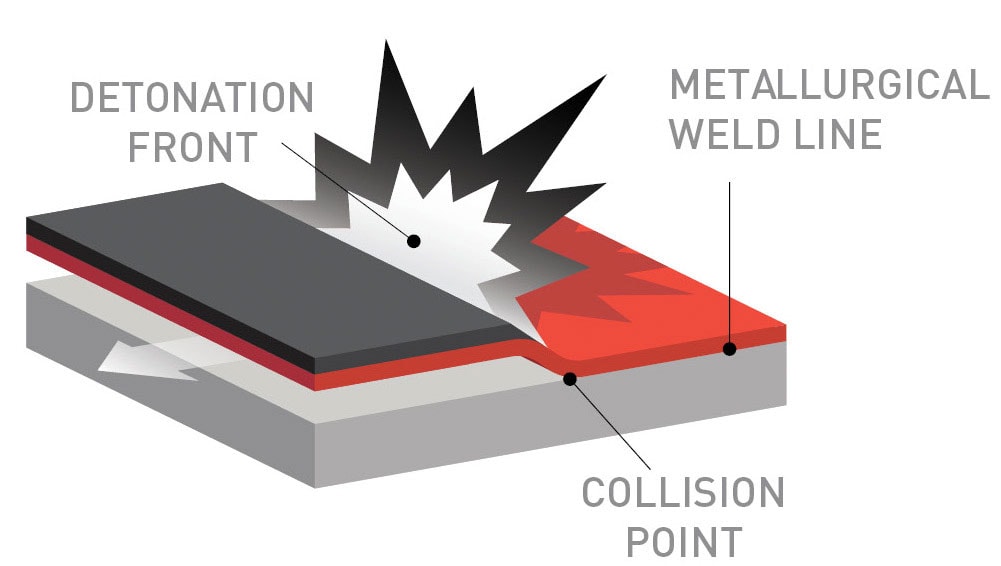
Figure 3: General arrangement of the plates during the welding process
Figure 3 shows the general arrangement of the plates during the welding process. The distance of the gap is one of the crucial elements in explosion welding, helping to determine the angle of impact, the velocity of impact, and the consistency of the jet [5]. DetaClad™ has been extensively used in Fertiliser and Chemical plants (such as Urea, Nitric Acid, Purified Terephthalic Acid, Acetic acid, PolyCarbonate, and Chlor-Alkali to name a few), refinery, LNG, geothermal, and nuclear applications.
NobelClad has recently launched a revolutionary reactive clad metal product known as DetaPipe™. DetaPipe is a mechanically clad pipe product. DetaPipe will be manufactured to B31.3 specifications which will assist the piping engineer when designing a piping system. Considering this is mechanically clad, NobelClad performs gripping force tests, like oil and gas Industry standard API 5L. In addition, the test spools have been tested at 200 bar and 225°C.

Figure 4: DetaPipe™ Zr, which is a pipe spool clad with zirconium.
In figure 4 the DetaPipe™ Zr, is a pipe spool clad with zirconium. This pipe spool, from flange face to flange face, is fully clad with zirconium inside carbon steel. This pipe spool is also available to be clad with Titanium. Pending needs, DetaPipe is fabricated with either ANSI flanges, compact flanges, or seal ring hubs. DetaPipe™ is currently being offered in straight lengths up to 9 meters and includes elbow options.
NobelClad can produce pipe spools currently from 6” to 30” NPS however flexibility exists to downsize or upsize these dimensions by a few inches depending on the application and business viability. We encourage users to reach out to NobelClad to discuss further such possibilities.
To solve for example the corrosion issues in the earlier discussed pipe reactor, NobelClad suggests using a DetaPipe™ Zr spool along with flange faces made with DetaClad™ Zr. NobelClad could produce the entire length of the pipe reactor with Zr mechanical cladded, however considering cost benefits, the solution proposed includes replacing only the section subject to maximum corrosion and erosion. Of all the corrosion-resistant metals, Zirconium or Tantalum are highly resistant to all forms of corrosion which include crevice corrosion [6]. Refer to figure 5.
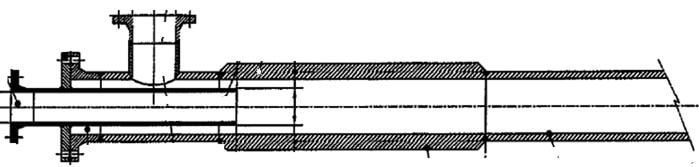
Before insertion of DetaPipe™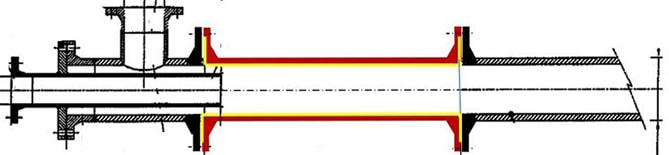 After insertion of DetaPipe™
After insertion of DetaPipe™
Figure 5: NobelClad solution for pipe reactors on ammonium- nitrate plants
Recently, NobelClad installed a Tantalum test spool for a Chlor-Alkali application. See figure 6. NobelClad is further working to develop tees and reducers.
For more details, please refer to the Deta221 specification for DetaPipe™. This specification can be downloaded upon request from NobelClad’s website: https://www.nobelclad.com/
Conclusions
Pipe reactors in Ammonium-Nitrate plants suffer from short lifetimes due to serious corrosion and erosion issues.
NobelClad’s DetaPipe™ coupled with its highly used and recommended DetaClad™ solution could provide a unique opportunity to address the problem faced by licensors and end-users in the pipe-reactors of ammonium-nitrate plants.
The NobelClad solution provides higher safety and reliability standards and less downtime and maintenance leading to an attractive pay-back time.
NobelClad’s DetaPipe™ has several possibilities for usage in mining, fertilizer, and other chemical applications. Presently, NobelClad is encouraging end- users and licensors to contact us to jointly evaluate test-spools in your critical applications.
Sources
- IFS Proceeding 515, Ammonium Nitrate production using a pipe reactor: Experiences over 10 years, Martin Voorwinden, Jean Bernard Peudpièce, Jean François Granger, 2003
- 2022, Ammonium-Nitrate and Fertilizer Safety, Kishore (Kish) D Shah MBE, 119-120
- Corrosion Engineering Guide, Giel Notten
- NACE Paper 03459 Recent Developments in Reactive and Refractory metal explosion clad technology, John G. Banker
- Explosion Welded Titanium, International Titanium Association – Titanium 2009, September 13-16, 2009, Waikoloa, Hawaii, USA, by: Michael Blakely, Dynamic Materials Corporation
- Zircadyne Corrosion Properties WAH CHANG Albany
- Deta-221 specification by Curtis Prothe and Brain Payton, NobelClad
Post Category
- News Article
Topic
- Maintenance
- Pressure Vessels
Published Date
December 12, 2024






